To define a temperature scale that does not depend on the thermometric property of a substance, Carnot principle can be used since the Carnot engine efficiency does not depend on the working fluid. It depends on the temperatures of the reservoirs between which it operates.
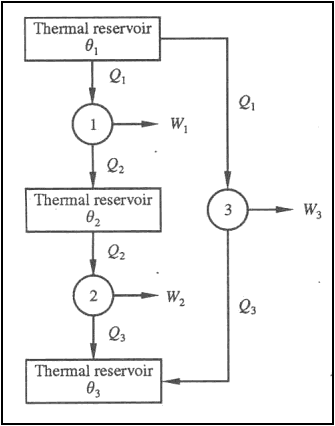
Consider the operation of three reversible engines 1, 2 and 3. The engine 1 absorbs energy Q1 as heat from the reservoir at T1, does work W1 and rejects energy Q2 as heat to the reservoir at T2.
Let the engine 2 absorb energy Q2 as heat from the reservoir at T2 and does work W2 and rejects energy Q3 as heat to the reservoir at T3.
The third reversible engine 3, absorbs energy Q1as heat from the reservoir at T1, does work W3 and rejects energy Q3 as heat to the reservoir at T3.
h1 = W1 / Q1 = 1- Q2/Q1 = f(T1,T2)
or, Q1/Q2 = F(T1,T2)
h2 = 1- Q3/Q2 = f(T2,T3)
or, T2/T3 = F(T2,T3)
h3 = 1- Q3/Q1 = f(T1,T3)
T1/T3 = F(T1,T3)
Then , Q1/Q2 = (Q1/Q3)/(Q2/Q3)
Or, F(T1,T2) = F(T1,T3) /F(T2,T3)
Since T3 does not appear on the left side, on the RHS also T3 should cancel out. This is possible if the function F can be written as
F(T1, T2) = f(T1) y (T2)
f(T1) y (T2) = {f(T1) y (T3)} / {f(T2) y (T3)}
= f(T1) y (T2)
Therefore, y (T2) = 1 / f(T2)
Hence, Q1 / Q2 = F(T1,T2) = f(T1)/ f(T2)
Now, there are several functional relations that will satisfy this equation.
For the thermodynamic scale of temperature, Kelvin selected the relation
Q1/Q2 = T1/T2
That is, the ratio of energy absorbed to the energy rejected as heat by a reversible engine is equal to the ratio of the temperatures of the source and the sink.
The equation can be used to determine the temperature of any reservoir by operating a reversible engine between that reservoir and another easily reproducible reservoir and by measuring efficiency (heat interactions). The temperature of easily reproducible thermal reservoir can be arbitrarily assigned a numerical value (the reproducible reservoir can be at triple point of water and the temperature value assigned 273.16 K).


Comments are closed.