The net rate of disappearance of A
Instantaneous selectivity
If α > β use high concentration of A. Use PFR.
If α < β use low concentration of A. Use CSTR.



Series Reactions (p. 283)
Example: Series Reaction in a batch reactor
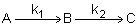
This series reaction could also be written as
Reaction (1)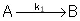 : -r1A=k1CA
: -r1A=k1CA
Reaction (2)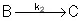 : -r2B=k2CB
: -r2B=k2CB
Mole Balance on every species
Concentration-Time Trajectories
Schemes for maximizing the selectivity for Van Der Vusse Kinetics
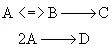















 in a CSTR
in a CSTR
Comments are closed.