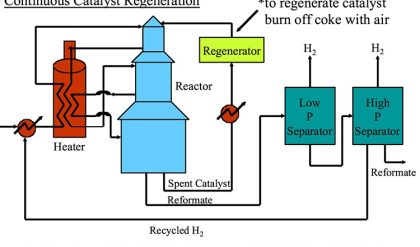
Although a number of different designs exist for the FCC process,a number of general principles can be described on the basis of Fig. 3. FCC, or at least the cracking reaction, is an endothermic process. The heat required for cracking is produced by sacrificing a small portion of the feedstock, and burning it in the regenerator.

Fig. 3 Schematic depiction of the fluid catalytic cracking (FCC) process, including reactor and regenerator.
Hot catalyst material is combined with pre-heated feedstock at the bottom of the riser reactor. The catalyst-to-oil ratio at the bottom of riser is larger than one, and a typical ratio is 5.5. The temperature at the bottom of the riser is typically in the range of about 550 °C. The reactant mixture expands due to the cracking reaction as gases are formed, and the catalyst/feedstock mixture is rapidly transported up the riser reactor, at speeds approaching 40 m s−1. The typical contact time in a riser is therefore in the order of seconds. At the top of the riser reactor, the temperature has dropped to about 500 °C as catalytic cracking is an endothermic process. The catalyst is separated from the product mixture and stripped of remaining useful product by steam treatment. The products are further refined downstream. The catalyst material, on which a certain amount of carbon, better known as coke, has been deposited during the cracking process, is transported to the regenerator, where the coke is burned off. The catalyst is thus regenerated and re-used continuously. Depending on the exact conditions (such as the oxygen availability), the regenerator temperature can reach up to 760 °C.
The selectivity to gasoline is in the order of 50% (see also Fig. 2). The catalyst temperature cycles between about 500 °C and about 760 °C, while it is moving at great speed. It is clear that this means the catalyst is exposed to harsh reaction conditions. As a result of this, the catalyst deactivates. A conservative estimate is that a typical FCC catalyst particle has an average lifetime in the order of about 1 month. Since it is not possible in the present process to selectively remove the deactivated catalyst, refiners remove a small portion of the complete inventory of the regenerator at fixed intervals (typically daily), and replace the removed catalyst with fresh catalyst. When this practice is performed for a longer period, a more or less steady state is reached in the catalyst life-time distribution, which is called equilibrium catalyst, or E-cat. Depending on the size of the FCC unit and the operational parameters, catalyst withdrawal rates can be between 1 and 30 tons per day.
The FCC unit in the oil refinery
The function of the FCC unit in an oil refinery is to convert heavy gas oil (HGO), vacuum gas oil (VGO) or residue feedstocks into useful products. Fig. 4, based on models by Fu et al. and Ma et al.,27 provides an artist impression of molecules such as could be found in an FCC feedstock, depicting larger aromatic structures with alkyl side-chains, as well as sulfur and nitrogen impurities (oxygen would be present in similar molecules), while Fig. 5a illustrates the complexity of a typical VGO feedstock with a GC × GC plot. When applying a zeolite Y-containing FCC catalyst material the wide variety of molecules present in the VGO feedstock is converted into molecules with on average a lower molecular weight, as illustrated in Fig. 5b, including molecules in the gasoline range (i.e., the 150 °C boiling temperature range). A typical molecule in the gasoline range would be 2,2,3-trimethylpentane (i.e., iso-octane). VGO feedstocks typically boil at 340–540 °C30 while resid has a higher boiling range (>540 °C), and contains multi-layered systems of poly-aromatic rings.

Fig. 4 Typical molecules that could be found in an FCC feedstock, depicting larger aromatic structures with alkyl side-chains, as well as impurities: in this case sulfur (yellow) and nitrogen (blue). Structures based on VGO-molecule cores described in Fu et al. and Ma et al. The structures were sketched in the ADF-builder, and energy-minimized using the built-in UFF force field in ADF.28 The resulting atomic positions were rendered with POV-Ray 3.6.

Fig. 5 (a) GC × GC plot of a typical FCC feedstock (i.e., VGO). (b) GC × GC plot of the products of cracking the VGO in (a), making use of an FCC catalyst with zeolite Y. (c) GC × GC plot of the products of cracking the VGO making use of an FCC catalyst with zeolite Y in the presence of zeolite ZSM-5 as additive.
In addition to multi-aromatic ring structures, both VGO and resid, also contain impurities, such as sulfur and nitrogen, and Ni, Fe and V. These are typically remainders from the plant or animal life forms that originally made up the organic matter that decayed into fossil fuels over millions of years, although they can also originate from the interaction of the oil fractions with rock formations. Interestingly, by comparing the GC × GC plots of Fig. 5b and c one can appreciate the influence of the addition of zeolite ZSM-5 to an FCC catalyst material.
A more schematic way of illustrating the FCC conversion process is shown in Fig. 6. Approximately 45% of the original feedstock (i.e., middle distillates, naphtha, and C2–C4-range molecules) can be further processed without conversion e.g. in reforming and isomerization to increase their value, and will likely require some form of hydrotreatment (e.g. HDS) to remove impurities.

Fig. 6 The effect of FCC conversion on total refinery product. Left: Atmospheric distillation frees up about 50% of the feedstock (middle distillates, gasoline and light gases). Heavy gas oil (HGO) and vacuum gas oil (VGO) are converted in the FCC unit. The products from FCC are combined with the initial products from crude distillation in the column on the right. More recent FCC processes will also convert part of the residue. Data from ref. 31.
A major part of the remaining relatively low-value bottom-of-the-barrel fractions (HGO and VGO in this example) are converted to desired products by the actions of the FCC catalyst, in which molecules are cracked to form high-octane rating products. The residue is not converted by the FCC catalyst in this particular example,31 although present day FCC catalyst materials can certainly convert resid, and resid FCC has now become an important process and, consequently, a vast amount of research is directed to focus on resid conversion.


Comments are closed.